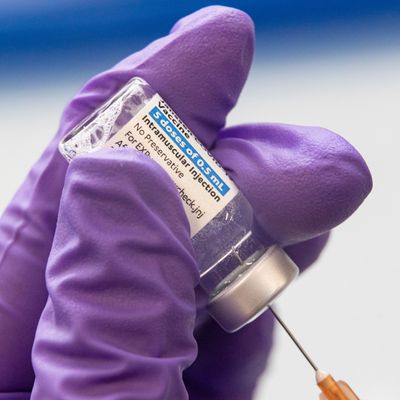
The one-shot Johnson & Johnson vaccine has been a critical boost in the plan to increase the pace of the COVID-19 vaccination effort, halving the number of doses for those who receive it and allowing health officials to easily inoculate older, homebound residents where they live. But that momentum will now slow down: Because of an error at a facility in Baltimore producing the Johnson & Johnson shot, some 15 million doses have been thrown out and further distribution of their vaccine candidate is temporarily on pause.
According to the New York Times and Politico, workers at a plant run by the pharmaceutical manufacturing company Emergent BioSolutions — which has partnered with both Johnson & Johnson and AstraZeneca — accidentally mixed up the active ingredients of the two candidates over the past few weeks. As a result, federal regulators have delayed authorization of the plant’s production lines. (The AstraZeneca shot has yet to be approved by the Food and Drug Administration.)
For those who have already received the Johnson & Johnson shot, worry not: All doses that have already been delivered and administered were developed in a Netherlands facility approved by regulators. But all future doses of the J&J shot are scheduled to come from the Maryland facility, meaning a significant delay for the firm which has signed a deal with the federal government to produce 100 million doses.
Federal officials still anticipate there are enough doses from the other authorized companies, Pfizer-BioNTech and Moderna, to achieve Biden’s goal of vaccinating every adult by the end of May. Once all three vaccine candidates are in distribution again, the Biden administration has secured enough doses to fully vaccinate 500 million people, well over the U.S. population of around 330 million.
It’s unclear how the suspension will affect the reputation of the Johnson & Johnson product, which has become the most popular option for many eligible for the vaccine, due to the convenience of getting just one dose. Because it does not require deep-freeze storage — as the other two approved candidates do — it is also much easier to distribute, allowing for state and city health departments to bring the shot to underserved communities. After the critical error, the Biden administration reportedly asked Johnson & Johnson to directly supervise the production at the Maryland facility.
While the Johnson & Johnson debacle is the most significant slowdown in the U.S. since vaccinations ramped up early this year, several European nations have suspended use of the AstraZeneca candidate for people under 60 after concerns that it may cause rare, but serious, blood clotting in younger patients.