Oral arguments have begun, with solicitor general going first on behalf of Biden administration
Oral arguments have begun. Solicitor General Elizabeth B. Prelogar is kicking them off, arguing on behalf of the Biden administration, and is allotted 20 minutes.
Jessica L. Ellsworth, a lawyer for Danco, which manufactures the brand name version of mifepristone, Mifeprex, will go next and is allotted 10 minutes for arguments.
Erin Hawley, wife of Sen. Josh Hawley, R-Mo., will represent the Christian legal group challenging access to mifepristone, and will have 30 minutes to deliver arguments for the respondents.
Rare all-women lineup will deliver arguments before the Supreme Court today
Notably, all lawyers arguing today in support of and against the FDA's actions to increase access to mifepristone are women — an uncommon scenario in oral arguments before the Supreme Court.
Solicitor General Elizabeth Prelogar will represent the FDA in support of its moves to make mifepristone more accessible. Lawyer Jessica Ellsworth will also represent drug manufacturer Danco Laboratories.
Arguing against the petitioners is Erin Hawley, the wife of Missouri Republican Sen. Josh Hawley, who is representing a group of doctors and other medical professionals opposed to the actions of the FDA on mifepristone access.
Crowds of protesters gather outside court, mainly in support of abortion rights
Different pockets of rallies formed outside the Supreme Court building. Most appeared to be in favor of abortion rights, chanting over megaphones, but there are smaller groups of people with signs protesting against “chemical abortions” as well. The energy outside of the courthouse is vibrant. There has been a huge turnout with some people even sleeping on the sidewalks in line as early as yesterday morning.
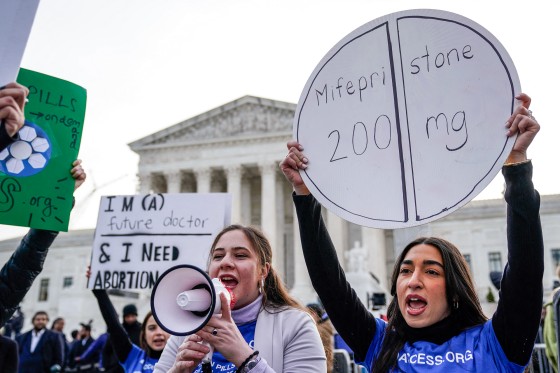

Biden campaign blames Trump for potential abortion medication restrictions
In advance of today's Supreme Court arguments, the Biden campaign pointed the finger at former President Donald Trump for the potential restrictions.
"This case could strip away access to medication abortion everywhere in this country," said Biden campaign manager Julie Chavez Rodriguez during a press call yesterday. "It would be the biggest step towards Donald Trump’s ultimate goal of a nationwide abortion ban since Roe was overturned."
Sen. Elizabeth Warren, who's also a member of the Biden campaign national advisory board, said the case before the Supreme Court is about Trump and the GOP pushing closer to a national abortion ban, rather than about the safety or efficacy of medication abortion.
"Donald Trump and MAGA Republicans are prepared to use every tool in their toolbox to control women’s bodies, banning abortion nationwide, ending access to IVF and even attacking contraception access," Warren, D-Mass., said on the call.
Lawyer for doctors' group played key role in Dobbs decision
Erin Hawley, who is representing the group suing the FDA, is senior counsel at the Alliance Defending Freedom, a conservative legal group that represents petitioners in many case against abortion. Hawley wrote briefs for the state of Mississippi in the Dobbs case, which overturned Roe v. Wade.
Hawley met her husband, Sen. Josh Hawley, R-Mo., while they were both clerks for Chief Justice John Roberts, who concurred with the majority opinion of Dobbs but stopped short of also reaching the decision to overturn Roe.
Abortion pill case could spark challenges to IVF, birth control
Vaccines, birth control pills, hormone therapies and fertility drugs would be subject to new litigation if the Supreme Court endorses a challenge to the abortion pill mifepristone, pharmaceutical industry experts warn.
When the court today weighs whether to roll back FDA findings that made mifepristone more readily available, it is not just access to that particular drug, used for the majority of abortions nationwide, that is on the line.
The pharmaceutical industry has raised the alarm, telling both the justices in court filings and anyone else who will listen that giving individual federal judges the power to cast aside the agency’s scientific health and safety findings would cause chaos within the sector. It would likely lead to litigation over other drugs, both current and those yet to be approved, on which people have strong feelings.
Will justices seize on the Comstock Act?
In response to the 2021 decision to allow mifepristone to be sent by mail, anti-abortion advocates have seized on a hitherto obscure 19th-century law called the Comstock Act, which prohibits the mailing of any drug or medicine that can be used for abortion. They argue that the Comstock Act should be taken into account in assessing the FDA’s decision to dispense with in-person visits.
When the 5th U.S. Circuit Court of Appeals ruled on the issue, one of the judges wrote that the FDA decision violated the Comstock Act, but the decision by the three-judge panel did not rely on that finding.
The FDA in court papers described it as a “doubly flawed” argument, arguing that the law was only intended to prohibit drugs that would lead to an “unlawful abortion,” not abortions that are lawful. The Justice Department’s Office of Legal Counsel issued an opinion in December 2022 that supports the Biden administration’s position.
Justices could decide the case without addressing FDA authority
The Supreme Court could rule for the government, leaving in place the current approvals for mifepristone, without deciding the knotty legal issues about the FDA approval process.
The government has argued strenuously that the doctors and others who filed the lawsuit do not have legal standing because they cannot show any injury that can be traced to the FDA’s decisions.
If the court were to adopt that argument, it could simply rule that the lawsuit should be dismissed.
The doctors themselves do not prescribe mifepristone, but they argue they are injured because they could be required to treat patients who have taken the pill and have serious side effects. As they oppose abortion, any actions they are forced to take to help a woman complete the process would make them complicit, the plaintiffs argued in court papers.
The FDA’s lawyers wrote in the government’s brief that the plaintiffs can at best point at a “hypothetical scenario,” which is not enough to establish standing.
The challengers, the brief stated, “cannot identify even a single case where any of their members has been forced to provide such care.”
Challengers say FDA decisions were 'arbitrary and capricious'
The challengers — doctors and other medical professionals who oppose abortion — argue that the FDA failed to sufficiently take into account safety concerns when the restrictions on mifepristone, including the requirement that the patient have an in-person visit with a doctor, were lifted.
Among other things, they note that the FDA conceded there would be an increase in emergency room visits by women suffering side effects as a result of the pill being made available by mail. As a result, the government acted in an “arbitrary and capricious” manner in violation of a law called the Administrative Procedure Act, the plaintiffs argue.
The FDA said in court papers that its actions were “supported by an exhausting review of a record including dozens of scientific studies and decades of safe use of mifepristone by millions of women.”
Doctors and patient advocates fear restricted access to abortion pill
About two years after the Supreme Court overturned Roe v. Wade, the court today will revisit the issue of reproductive rights, this time contemplating whether to limit access to mifepristone, the first of two pills used in medication abortion.
Ahead of oral arguments and eventual ruling, doctors and patient advocates are expressing alarm about what might happen if the high court decides to tighten access to the drug.




